Just the facts
What dairy processors must know about FDA's new Nutrition Facts label
Dairies and other food makers must comply with the new FDA Nutrition Facts label rules by July 26, 2018. Get started now.
Dairy food companies must get ready now for the sweeping changes that will force all food companies to overhaul their nutrition facts label in the coming years. An estimated 800,000-plus labels from across the food industry will need to change nutrition information, graphic design and layout to meet the Food and Drug Administration’s new regulations.
FDA released its much-anticipated nutrition labeling revisions in May. These are viewed as one of the key regulatory legacies of President Obama’s administration and represent the most significant changes to the nutrition labeling requirements since they were first finalized more than 20 years ago. Recognizing a shift in the average American’s consumption habits and the need to update nutrition information, FDA’s two final rules changed the content and format of the Nutrition Facts label as well as to the reference amounts that determine the serving sizes of foods and beverages.
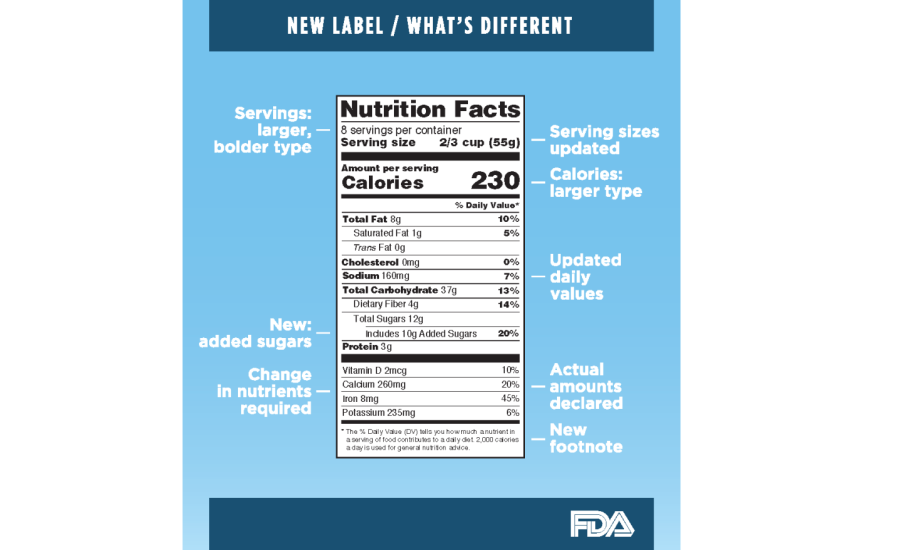
Here is a quick look at what the new labeling requirements mean for dairy companies and their products.
A refreshed design for the Nutrition Facts label
The iconic look of the label remains, but FDA updated it to ensure consumers have access to the information they need to make informed decisions about the foods they eat. To emphasize that, for healthy weight management, calories count, FDA increased the prominence for “Calories” and required a larger type for the “Serving Size” declaration
Vitamin D and potassium replace vitamins A and C as required on the label. With the change, manufacturers must declare the actual amount, in addition to percent Daily Value of vitamin D, calcium, iron and potassium. The footnote is changing to better explain what percent Daily Value means.
The Nutrition Facts label must now declare both “Added Sugars” and “Total Sugars” in place of the current declaration for “Sugar.” The percent Daily Value for added sugars will be set at 50 grams per day, which aligns with the recommendations of the 2015 Dietary Guidelines for Americans.
At IDFA’s request, FDA refined the definition of added sugars to exclude dried and concentrated dairy ingredients. The rule states “dairy ingredients, even though high in lactose will not be considered as a source of ‘added sugars.’ However, lactose in its pure form when added to food, including other dairy ingredients, will count as ‘added sugars.’”
While continuing to require Total Fat, Saturated Fat and Trans Fat on the label, Calories from Fat is being removed, because research shows the type of fat is more important than the amount.
Daily values for nutrients such as total fat, sodium, dietary fiber, potassium, calcium and vitamin D are being updated based on newer scientific evidence. These changes may alter the ability of certain dairy products to make nutrient content claims like “good source of potassium” or “excellent source of vitamin D.”
Updated serving sizes for ice cream
FDA requires serving sizes be based on the amount of foods and beverages that people actually eat, not what they should eat. For example, the reference amount used to set a serving of ice cream was previously one-half cup but will be changed to two-thirds cup. Initially, FDA proposed the serving for ice cream be increased to 1 cup, but IDFA succeeded in getting this revised. Yogurt’s serving size will reduced from 8 ounces to 6 ounces.
Package size can affect how much people eat. So containers with less than two servings will now be labeled as a single-serving container. Certain products that are larger than a single serving but that could be consumed in one sitting or multiple sittings (meaning, at least two and up to three servings) will be required to bear dual-column labeling to provide information based on both the serving size and the entire container. This will impact the labeling of products like a pint of ice cream or a 16-ounce container of milk.
These changes mean that dairy and ice cream companies may need to revise their formulas, increase fortification or remove claims to be compliant.
It’s not too early to start. Engage your team of labeling, formulation, marketing, and purchasing experts to work with packaging and label companies to develop a comprehensive plan to change labels. The compliance date is July 26, 2018 for manufacturers with more than $10 million in annual food sales (July 26, 2019 for manufacturers with less than $10 million in annual food sales).
IDFA will be assisting the dairy industry with educational resources, webinars and manuals on the details of the regulations to provide the facts you need to make compliant and effective labels.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








