Globule warming
Why ice cream melts
By understanding melting characteristics, ice cream manufacturers can create products with the mouthfeel of full-fat products but without the calories.


Some ice creams melt and collapse quickly. In Figure 1 you can see the complete drip-through of a U.S. commercial ice cream product after 70 minutes..

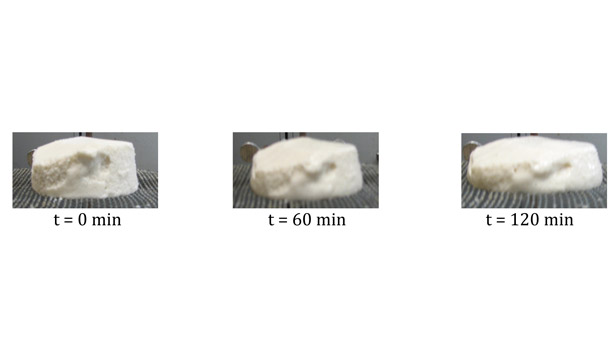
Some ice creams completely retain their shape, appearing to have little to no melt. Figure 2 shows an example of a U.S. commercial ice cream sample with resistance to collapse even after 120 minutes.



Only on the hottest summer days or when we’ve been unexpectedly called away from our scoop do we experience ice cream melting in some way other than in our mouths. However, the meltdown of ice cream is an important facet of ice cream science, one that is currently under study in the Frozen Dessert Center at the University of Wisconsin-Madison.
Ice cream is a complex frozen dessert typically made from milk fat (at least 10% for the United States), sweeteners (typically sucrose), stabilizers and emulsifiers. This mix, after being pasteurized, homogenized and aged, is then frozen in a scraped-surface heat exchanger.
During freezing, ice crystals are formed, of course, but air is also incorporated and the phenomenon of partial-coalescence of fat globules takes place. As we’ll see, controlling the fat globules is key to controlling melting.
Why would ice creams melt in different ways? To study this, the meltdown of ice cream is quantified by placing an 80-gram slab sample on a mesh screen and allowing it to melt and/or collapse at ambient temperatures for up to two hours. The drip-through weight is recorded along with an image of the ice cream during melting. (See images, above.)
From the drip-through test, we can obtain much information about the ice cream products. Some commercial ice creams melt and collapse quickly while others melt and completely retain their shape, with little to no drip-through.
What is the science behind this? How can one ice cream remain standing while another one, with essentially the same ingredients, completely collapse and flow through the screen? The answer lies within the microstructure of ice cream, primarily with the fat globules.
Ice cream is a fascinating and extremely complex product, in part because of its microstructure. Frozen ice cream is composed of ice crystals, air cells, individual fat globules and partially coalesced fat globule clusters surrounded by a serum phase.
In ice cream mix, individual, partially crystalline fat globules of about 1 µm in size are stabilized by the milk proteins. Emulsifiers are added to the mix to actually help destabilize the fat globules, primarily during freezing. Under the high shear conditions in the scraped-surface freezer, some of the individual fat globules begin to coalesce, but the crystalline fat within the globules arrests coalescence at some intermediate state. The result is creation of fat globule clusters ranging in size typically from about 10 to 100 µm.
This process of forming clusters from individual fat globules is often called partial coalescence, which is influenced by formulation factors (for example, emulsifier type and content) and processing conditions (shear rate during freezing). These clusters are an important key to how ice cream melts.
The different ratios of the microstructure components in ice creams dictate their drip-through rate and collapse. In particular, it’s the number density of clusters above some critical size that determines meltdown. For example, the ice cream in our first sample (with 10% fat and less than 10% partial coalescence) has fewer and smaller clusters than the second sample (with 14% fat and more than 30% partial coalescence). The former collapses right through the screen while the latter retains its shape and resists collapse.
In principle, this is related to the ability of large clusters to jam as the ice melts and the system starts to collapse. Melting ice leads to flow of diluted serum through the spaces between air cells and fat clusters. If the fat clusters are small and few in number, they flow with the serum between the air cells and the entire system collapses. If the clusters are large and numerous, they get stuck on each other (they jam) as the system begins to collapse, providing a network-like structure that prevents any further collapse.
How do these meltdown studies relate to our perception of ice cream melting in our mouths (sensory melt rate)? Will the average consumer notice that one ice cream melts and collapses faster in the mouth than another?
In a sensory study, panelists could not distinguish the difference in melt rate of the two ice cream samples. This is probably because we experience ice cream differently in the mouth than when it sits out at ambient temperatures.
The mouth is naturally warmer than that of ambient temperatures and when eating, natural chewing motions occur, even with ice cream. This leads to complete destruction of the microstructure and little room to explore the true melting of the product inside the mouth. Our mouths also have varying saliva, which can break down ice cream differently depending on the composition.
Many consumers enjoy eating ice cream on a cone (thank you 1904 World’s Fair!). Maybe ice cream dripping off a cone correlates better with the meltdown test at ambient temperatures. Although formal studies have yet to be done on this, it stands to reason that the ice cream in our first sample would drip considerably more (and faster) from a cone than would the other ice cream sample.
On a hot summer day, you’d be licking furiously on the first ice cream to keep ahead of the drips, whereas you could eat the second ice cream more leisurely without worrying about losing product or getting sticky hands.
How can we look at meltdown rate differently so that it correlates with what happens in the mouth? This is a question that we are beginning to explore to better understand and make correlations with the melting behavior of frozen desserts. In fact, the entire field of food oral processing, where physics and physiology meet psychology, addresses exactly these questions.
Maybe a mechanical mouth model could be used to measure how ice cream melts in the human mouth. And maybe this could be used to design complex microstructures that deliver all the benefits of eating ice cream without all the decadence.
Although there is no “right” or “wrong” or “better” or “worse” when it comes to partial coalescence in ice cream, manufacturers can use it to their advantage. If desired, they can control the formation of fat globule clusters to deliver ice cream products (low to full fat) with particular melt and sensory properties, providing consumers with a desirable mouthfeel regardless of fat content.
Sure, melting ice cream seems as simple as it is inevitable. But as with most things, when looked at more closely, a complex set of interactions is revealed, which in this case, leads to highly unpredictable meltdown and sensory perception.
So the next time you have ice cream, whether a scoop or a swirl, do a little science of your own as you observe its melt rate both at ambient conditions and in the mouth.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!